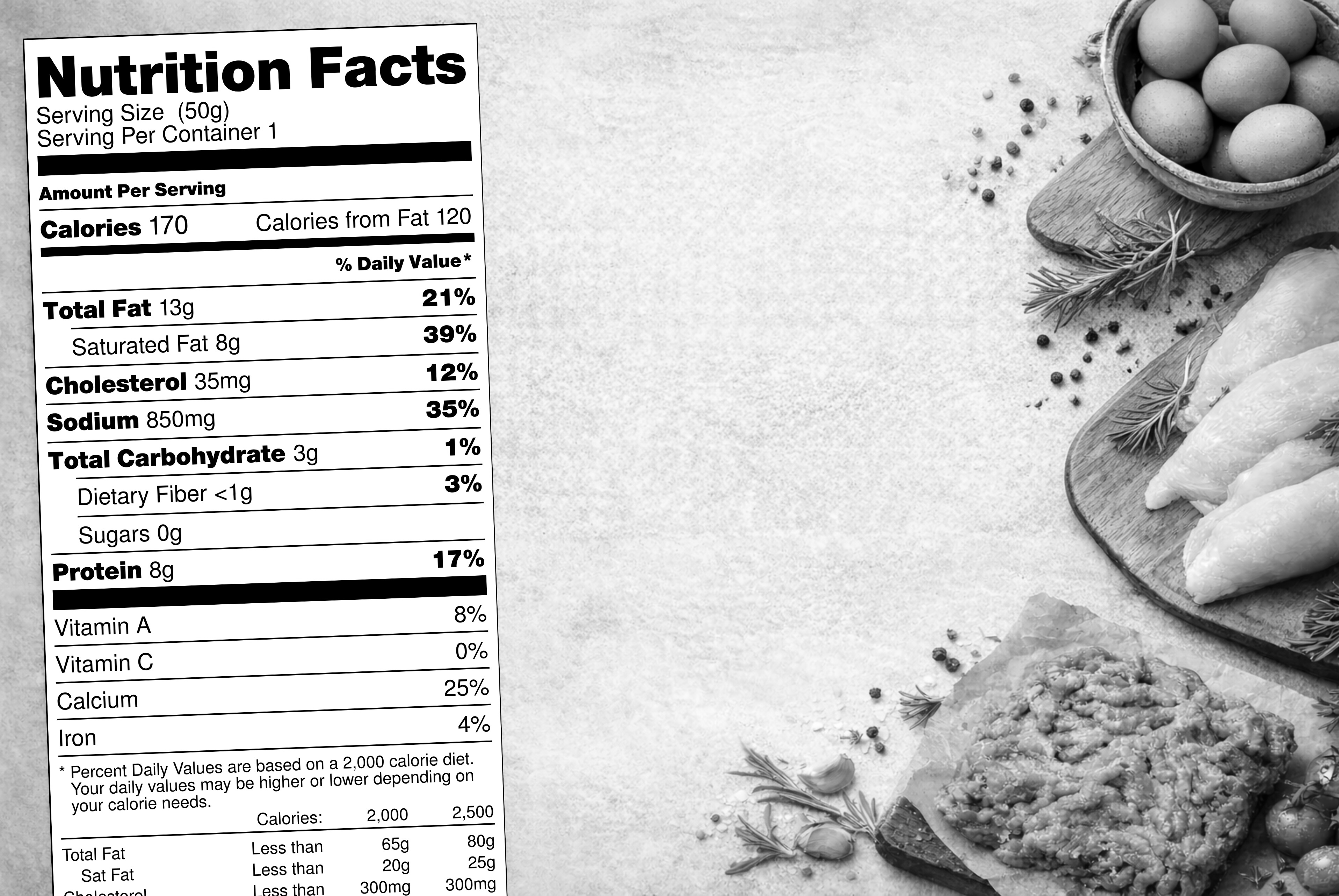
FDA Updates Definition of “Healthy” for Food Labels
The U.S. Food and Drug Administration (FDA) has finalized new criteria for the use of the “healthy” on food labels, which companies will be required to comply with by February 25, 2028. This update, part of the Biden Administration’s broader initiative to combat chronic disease, aims to align the criteria with current nutrition science, the Dietary Guidelines for Americans, 2020–2025, and the updated Nutrition Facts label.
Key Updates in the Final Rule
The FDA has maintained its proposed framework but made several changes to accommodate stakeholder feedback. These updates include:
- Expanded List of Automatically Qualifying Foods: Foods such as vegetables, fruits, whole grains, low-fat dairy, seafood, nuts, seeds, and beverages with fewer than five calories per serving now qualify for the “healthy” claim without meeting additional nutrient or food group requirements.
- Greater Flexibility for Small-Portion Foods: Foods consumed in small quantities (e.g., less than 50 grams or 3 tablespoons) have more leeway to qualify.
- Revised Nutrient Limits: The baseline limits for saturated fat, sodium, and added sugars have been adjusted across various food categories to reflect their role in healthy dietary patterns. For example, dairy products now have a higher saturated fat limit (10% of the daily value), while oils and oil-based dressings have a total fat limit of 20%.
- The full details of the final rule for using “healthy” on food labels can be found on the federal register website.
Background and Stakeholder Input
The final rule replaces the outdated 1994 regulation established under the Nutrition Labeling and Education Act. The previous rule, outlined in 21 C.F.R. 101.65(d)(1), focused on minimum nutrient content thresholds, while the new criteria adopt a food-group-based approach. Foods must now provide a minimum amount of a recommended food group (e.g., fruits, vegetables, whole grains) and adhere to limits for saturated fat, sodium, and added sugars.
Stakeholders, including industry groups, academics, and public health advocates, submitted approximately 400 comments. While there was general support for the updated definition, industry groups requested more flexibility, particularly for nutrient-dense foods like canned fruits, yogurt, and whole-grain bread. The FDA addressed some of these concerns by broadening the range of qualifying foods and adjusting nutrient thresholds.
Scope and Recordkeeping Requirements
The FDA has clarified that the term “healthy” applies to both the word itself and derivative terms like “healthier” or “healthfulness” when used in a nutritional context. Terms such as “nutritious” and “wholesome” are not regulated under the new rule but remain subject to general false advertising laws.
Manufacturers must maintain records to verify compliance with the criteria for “healthy” claims. These records, such as ingredient weight data and supplier certificates, must be available for FDA inspection.
Implications for the Food Industry
The updated rule offers flexibility for a broader range of foods to bear the “healthy” label, reflecting the FDA’s effort to promote nutrient-dense, accessible, and culturally relevant options. However, some industry concerns remain unaddressed, leaving the potential for legal or legislative challenges.
ENTR’s formulation and packaging compliance software can assist manufacturers with record keeping for all formulas where they want to make a ‘healthy’ claim, ensuring compliance with FDA requirements while simplifying the documentation processes.
The new rules are an important step in guiding consumers toward healthier choices and addressing diet-related chronic diseases, but its long-term impact will depend on how manufacturers and stakeholders adapt to the changes.